When it works, CAR-T treatment for cancer can seem like a miracle. With a single infusion of their own immune T-cells that have been genetically changed to find and kill cancer in the blood, about half of the patients with leukemia, lymphoma, and myeloma have a full remission, which is a functional “cure.” Acute lymphoblastic leukemia is the most common type of cancer in children, and CAR-T treatment has shown that it can cure up to 90% of these cases. Adult men with end-stage chronic lymphocytic leukemia were the first two people to get CAR-T treatment in 2010. They were still in remission a decade later.
Since 2017, the U.S. Food and Drug Administration has approved six CAR-T medicines, all of which are used to treat blood cancers. (Many studies are looking into how CAR-T therapy could be used to treat solid tumors, but none have reached the human trial stage yet.) Gilead Sciences’ Kite Pharma made $1.5 billion from just two of these drugs, Yescarta and Tecartus, in 2022.


CAR-T therapies were largely used as a last resort until recently. CAR-T may operate well earlier in the healing process. Last year, Yescarta was approved for second-line large B-cell lymphoma. Drugmakers already struggle to meet demand.
In 2022, Mayo Clinic researchers reported that the average wait time for CAR-T therapy was six months, and only 25% of those on the list received it. (Another 25% joined a clinical trial for an unapproved medicine.) Bristol Myers Squibb, Gilead’s Kite Pharma, and Novartis have experienced problems producing CAR-T medicines in recent years. Due to production issues, J&J and Legend Biotech delayed the U.K. introduction of Carvykti in March.
Autologous CAR-T infusions
Autologous CAR-T infusions are “living medicines” customized for each patient. Blood is usually drawn in hospitals or other facilities. T cells are removed and refrigerated or frozen in a central biomanufacturing center, where they are genetically “reprogrammed” to express a tumor-seeking molecule. This is a CAR. To ensure a therapeutic amount, transformed cells are “expanded” in an incubator for a few days or weeks. After testing, the cells are frozen and sent to the hospital for the patient. The surgery takes two to eight weeks “vein to vein.”
Prices for CAR-T treatments run from high $300,000 to high $400,000 on the list. Travis Young, VP of Biologics at the non-profit California Institute for Biomedical Research (Calibr), says, “It’s so expensive because every step in the process of making it has to be very well managed.
It needs a worker with a lot of training, clean rooms, and the infrastructure for shipping and freezing, and the most time-consuming part is testing the product before it goes on the market to make sure it is safe and effective. There are many ways for things to go wrong. “The supply chain is in its infancy,” says Young. “And it’s not just the buildings; it’s also how many people know how to do this.”
Companies are working on these problems in different ways to make them easier, faster, and cheaper to solve so that more people can get CAR-T treatments. One of the least likely candidates is Galapagos NV, a company based in Belgium. In June of last year, they announced a bold plan to make these expensive therapies faster and cheaper by developing them at the point of care, using a small, highly automated device about the size of a home microwave.
Galapagos never used CAR-T
Galapagos had never worked with CAR-T before, and since it was founded in 1999, it has only brought one product to market in Europe, the UK, and Japan: Jyseleca, a medicine for ulcerative colitis and rheumatoid arthritis that made sales equal to $95 million in the U.S. in 2022. And recently, a number of drugs for osteoarthritis and idiopathic pulmonary fibrosis failed in human trials. But it has a secret weapon in the form of its new CEO, Paul Stoffels, who used to be Johnson & Johnson’s top scientific officer.
Stoffels had one of the most impressive records in Big Pharma when he left Johnson & Johnson at the end of 2021. He says he did this to be closer to his children and grandkids in Europe. In his nine years as CSO and two years as global head of pharma R&D, the Belgian-born doctor and infectious disease expert-led teams that released 25 new medicines, including two blockbuster cancer drugs, groundbreaking treatments for HIV and tuberculosis, and vaccines for Ebola and COVID-19. Stoffels was in charge of making Carvykti, J&J’s CAR-T drug.
It was approved a few months after he left the company. During Stoffels’s time as CEO, J&J’s pharmaceutical sales went from $22.5 billion in 2009 to $45.6 billion in 2020, which is more than double. Seven of the drugs made through Stoffels have been added to the Essential Drugs List of the World Health Organisation. This means that they are considered essential for keeping health systems running well.
Stoffels is back
With this job, Stoffels is back in his home country and at a company, he helped start in 1999. And it has given him a chance to make changes that he couldn’t make at a bigger business. As soon as he became CEO in April of last year, he made a big change. He bought two companies that were working on different parts of CAR-T medicines and manufacturing, and then, four months later, he fired 200 people who were working on older drug programs.
Stoffels said the company needed to change following past issues. He explains, “We were concentrating on new targets and small-molecule drugs.” Starting from scratch takes 15 years to reach the market. We couldn’t do that. Today, people receive medicines differently. Why not start oncology with CAR-Ts and a system that will revolutionize everything?”
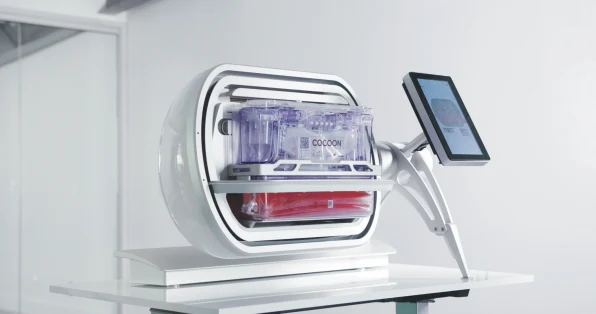
Due to a deal with Gilead, Galapagos had about $4.5 billion in cash on its balance sheet. Stoffels was able to buy the company a head start with this money. A Dutch company called CellPoint was already working on a cell-manufacturing platform called the Cocoon, which was a self-contained lab in a box that promised a remarkable seven-day vein-to-vein time. It was also running clinical trials of two potential CAR-T therapies for recurrent/refractory non-Hodgkin’s lymphoma and chronic lymphocytic leukemia.
Stoffels made an initial investment in CellPoint, and then in June of last year, he paid $130 million in cash to buy the company outright, with possible milestone payments of up to $105 million. At the same time, he gave $14 million to a company in Pittsburgh called AboundBio. This company works on antibody technology that could help engineer new receptors to target other kinds of cancer and make present treatments more effective.
Galapagos sets up manufacturing units
Galapagos expanded CellPoint’s Phase 1/2 clinical trials. It is testing two CAR-T treatment options and Cocoon production in six European facilities for safety, efficacy, and viability. Treatment response and vein-to-vein time are accurate. Stoffels wants 15 venues in eight European countries tested by year’s end. Galapagos plans more CAR-T cancer treatments next year. It wants to commence critical U.S. research in Q1 2024.
These investigations need more work and different expertise than standard drug trials, which involve producing and administering the medicine. Galapagos builds factories in its hospital partners, according to Stoffels. “That requires a lot of people” to train, equip, and test the production process. It’s difficult because it’s new, but it’s a lot simpler than centralized manufacturing, which entails spending a few hundred million dollars on a building, hiring 500 to 1,000 people, educating them, and making the goods there. We had already done the heavy science job.”
Never-frozen patient cells are better biologically. Stoffels believes that “fresh” Cocoon-made CAR-T cells multiply quickly and steadily in patients. This decreases CAR-T therapy’s cytokine release syndrome, which produces fevers, nausea, and exhaustion from immunotherapy overreaction. “The fact that this can be done in seven days means that people with a very short life expectancy can still get this kind of therapy,” he says. First Cocoon system CAR-T cell recipient responding well. Breathing troubles and a growing tumor sent him to the hospital. Centralized CAR-T manufacture prevented this.”
Stoffels says that “decentralization, simplification, and automation of the whole process will reduce the cost of CAR-T significantly,” but he won’t say by how much. “A lot of the cost of CAR-T treatments comes from time and work. If you put four or five Cocoon units in a hospital room, you can help 200 patients per year with just a few people.”
Galapagos is up against a lot of rivals
Even so, Galapagos is up against a lot of rivals. ClinicalTrials.gov lists 1,128 CAR-T therapy clinical studies, of which 275 are either recruiting patients or have finished Phases 2 or 3. A few other companies are also working on systems like the Cocoon. For example, the CliniMACS Prodigy device, which can also be used in the centralized facility and is in Phase 1/2 studies in the U.S. for patients with B cell Acute Lymphoblastic Leukaemia, is one of these systems.
Allogene, Precision BioSciences, and Caribou Biosciences are developing “off-the-shelf” allogeneic CAR-T therapies to eliminate waiting times. In vivo CAR-T may become mainstream. The therapy will directly modify T-cells or other immune cells with DNA or RNA instead of producing CAR-T cells outside the body. Ensoma, Vector BioPharma, Umoja Biopharma, Interius BioTherapeutics, Capstan, and Moderna work in this area.
Galapagos will face chemical component and raw material shortages like other enterprises. Distributed manufacturing also has issues, according to Young from Calibr: “All of the problems that come from not having enough highly trained technicians to do this are made worse if you are working across multiple hospital centers.” Since they don’t do much handwork, techs need less training. But sending this to all these care centers loses some control.”
But Stoffels has done the impossible many times before and doesn’t seem to be discouraged. He says, “I’ve spent my whole life trying to make medicines easy to get.” “That’s also one of our goals here. Because of the new science, we can do new, hard, and different things. And you’ll never get there if you don’t start.”